Health & Safety

NanoGUNE is committed to safety at work. All measures legally required have been implemented and are bound to all employees (Law 31/1995 on the Prevention of Occupational Risks, RD 39/1997).
The Facilites Manager, Gorka Arregui (g.arregui@nanogune.eu), is the Emergency Coordinator in nanogune. Raquel Olmos (r.olmos@nanogune.eu) is the interlocutor of Health and Safety. Together, with the collaboration of IMQ Prevención that has been designated as the external prevention service, coordinates the health and safety at nanoGUNE.
Health & Safety

NanoGUNE is committed to safety at work. All measures legally required have been implemented and are bound to all employees (Law 31/1995 on the Prevention of Occupational Risks, RD 39/1997).
The Facilites Manager, Gorka Arregui (g.arregui@nanogune.eu), is the Emergency Coordinator in nanogune. Raquel Olmos (r.olmos@nanogune.eu) is the interlocutor of Health and Safety. Together, with the collaboration of IMQ Prevención that has been designated as the external prevention service, coordinates the health and safety at nanoGUNE.

Prevention plan
The Occupational Risk Prevention Plan is a tool to bring together the preventative actions undertaken by CIC nanoGUNE and to establish the Occupational Risk Prevention Policy. Moreover, the H&S staff roles are described in the following documents.

Prevention plan
The Occupational Risk Prevention Plan is a tool to bring together the preventative actions undertaken by CIC nanoGUNE and to establish the Occupational Risk Prevention Policy. Moreover, the H&S staff roles are described in the following documents.

Risk Assessment
Risk assessment is a systematic process of identifying hazards and evaluating any associated risks within a workplace, then implementing reasonable control measures to remove or reduce them. This document evaluates the possible physical, chemical and ergonomical risks in each laboratory or workspace at nanoGUNE.
In the following link, you will find the risk assessment documents from all laboratories.

Risk Assessment
Risk assessment is a systematic process of identifying hazards and evaluating any associated risks within a workplace, then implementing reasonable control measures to remove or reduce them. This document evaluates the possible physical, chemical and ergonomical risks in each laboratory or workspace at nanoGUNE.
In the following link, you will find the risk assessment documents from all laboratories.

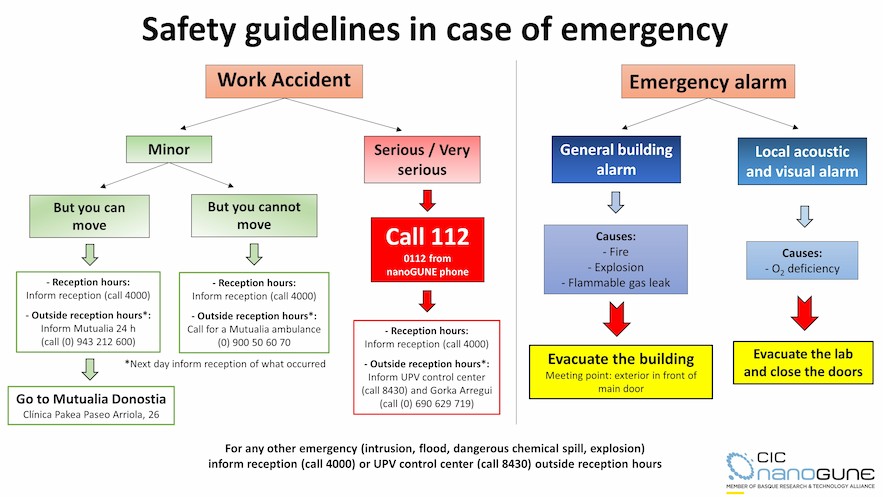
Emergencies
The purpose of the nanoGUNE Emergency Plan is the development, implementation and maintenance of the measures needed in the event of emergencies, as established in article 20 of the Occupational Risk Prevention Law (31/1995). This plan is defined as the organization of available human and material resources to control emergency situations that may cause serious damage to people or facilities. It is considered mandatory knowledge for all staff, who will try to interpret and apply it with the utmost efficiency, to avoid or mitigate risks, all within a framework of full collaboration.
You will also find the documents containing basic safety guidelines in case of emergency, how to act in case of illness or working accidents, as well as protocols of the use of products for chemical splashes.
In case of a working accident, the health insurance for all nanoGUNE employees is MUTUALIA Donostia (Paseo Arriola, 26). You must go there, prior information to reception staff or Gorka Arregui. Guest researchers must follow the guidelines of their employer or home institution.

Emergencies
The purpose of the nanoGUNE Emergency Plan is the development, implementation and maintenance of the measures needed in the event of emergencies, as established in article 20 of the Occupational Risk Prevention Law (31/1995). This plan is defined as the organization of available human and material resources to control emergency situations that may cause serious damage to people or facilities. It is considered mandatory knowledge for all staff, who will try to interpret and apply it with the utmost efficiency, to avoid or mitigate risks, all within a framework of full collaboration.
You will also find the documents containing basic safety guidelines in case of emergency, how to act in case of illness or working accidents, as well as protocols of the use of products for chemical splashes.
In case of a working accident, the health insurance for all nanoGUNE employees is MUTUALIA Donostia (Paseo Arriola, 26). You must go there, prior information to reception staff or Gorka Arregui. Guest researchers must follow the guidelines of their employer or home institution.

Medical check up
Medical examinations are annually carried out to provide periodic monitoring of the current state of the workers’ health based upon the risks inherent to their position, doing so while respecting people's right to privacy and dignity and while upholding the confidential nature of all information relating to workers' state of health. Our preventive health surveillance service (IMQ Prevención) will carry out health monitoring of all nanoGUNE employees. Administration staff will organize the periodic blood extraction and subsequent medical checkup. Guest researchers must follow the guidelines of their employer or home institution as they are not covered by this service.
In case you want to refuse the annual medical examination, you must sign and send the document you will find in the following link to Gorka Arregui (g.arregui@nanogune.eu).

Medical check up
Medical examinations are annually carried out to provide periodic monitoring of the current state of the workers’ health based upon the risks inherent to their position, doing so while respecting people's right to privacy and dignity and while upholding the confidential nature of all information relating to workers' state of health. Our preventive health surveillance service (IMQ Prevención) will carry out health monitoring of all nanoGUNE employees. Administration staff will organize the periodic blood extraction and subsequent medical checkup. Guest researchers must follow the guidelines of their employer or home institution as they are not covered by this service.
In case you want to refuse the annual medical examination, you must sign and send the document you will find in the following link to Gorka Arregui (g.arregui@nanogune.eu).

Maternity
There is a procedure for all female employees at nanoGUNE, to protect those in the gestation period, who have given birth or who are in the breastfeeding period, and their unborn/newborn/nursing infant(s) in compliance with Law 31/1995 on the Prevention of Occupational Risks, RD 39/1997.
After communicating a situation of pregnancy or breastfeeding, the risks associated with the job position must be determined and assessed to evaluate whether an adaptation or change of the job position is possible. If this is not possible, an application for financial assistance due to occupational risk will be submitted to the mutual insurance company.
Pregnant guest researchers must follow the guidelines and procedures stablished by their employer or home institution for these situations.
Maternity
There is a procedure for all female employees at nanoGUNE, to protect those in the gestation period, who have given birth or who are in the breastfeeding period, and their unborn/newborn/nursing infant(s) in compliance with Law 31/1995 on the Prevention of Occupational Risks, RD 39/1997.
After communicating a situation of pregnancy or breastfeeding, the risks associated with the job position must be determined and assessed to evaluate whether an adaptation or change of the job position is possible. If this is not possible, an application for financial assistance due to occupational risk will be submitted to the mutual insurance company.
Pregnant guest researchers must follow the guidelines and procedures stablished by their employer or home institution for these situations.

Coordination of Business Activities
The Coordination of Business Activities is established as the control measures for the prevention of occupational risks when workers from two or more companies are present at the same workplace. These measures are aimed at fostering cooperation between all the companies and workers involved. The goal is to ensure that such concurrent activities do not negatively impact the safety and health of the workers.
The Coordination of Business Activities is regulated by Law 31/1995 (RD 171/2004) and supports the prevention of occupational risks arising from business contracting and subcontracting. We refer to coordination when different companies share the same workplace. This must be done when clients, suppliers, maintenance technicians, external researchers and collaborators are going to carry out any activity at CIC nanoGUNE workplace.
In the following link, you will find the procedure for the coordination of business activities in nanogune.
Coordination of Business Activities
The Coordination of Business Activities is established as the control measures for the prevention of occupational risks when workers from two or more companies are present at the same workplace. These measures are aimed at fostering cooperation between all the companies and workers involved. The goal is to ensure that such concurrent activities do not negatively impact the safety and health of the workers.
The Coordination of Business Activities is regulated by Law 31/1995 (RD 171/2004) and supports the prevention of occupational risks arising from business contracting and subcontracting. We refer to coordination when different companies share the same workplace. This must be done when clients, suppliers, maintenance technicians, external researchers and collaborators are going to carry out any activity at CIC nanoGUNE workplace.
In the following link, you will find the procedure for the coordination of business activities in nanogune.

Standard Operating Procedures
A Standard Operating Procedure (SOP) is a set of established instructions or guidelines that describe the specific steps and processes to be followed to perform tasks consistently and efficiently, ensuring compliance with all safety measures within nanoGUNE facilities.
A new SOP is required when new working procedures that can entail additional risks that are not collected in the risk assessment document are going to be carried out at nanoGUNE facilities. In these cases, it is very important to describe in advance the working procedure and the emergency measures as well as the potential hazards.
In the following link you can find the list of updated SOPs and the template to fill in.

Standard Operating Procedures
A Standard Operating Procedure (SOP) is a set of established instructions or guidelines that describe the specific steps and processes to be followed to perform tasks consistently and efficiently, ensuring compliance with all safety measures within nanoGUNE facilities.
A new SOP is required when new working procedures that can entail additional risks that are not collected in the risk assessment document are going to be carried out at nanoGUNE facilities. In these cases, it is very important to describe in advance the working procedure and the emergency measures as well as the potential hazards.
In the following link you can find the list of updated SOPs and the template to fill in.

Biological safety level (BSL) 1 & 2 laboratories
At nanoGUNE, we have facilities classified as BSL 1 and BSL 2 in which we can work with biological agents of these categories.
BSL1-enabled laboratories are Labs 7, 8, 9, 10, 15, 23 and Eukaryote Culture Room, while BSL 2 agents can only be handled in Laboratory 4 and in the Bacteria Culture Room (within the Culture Room Facilities).
The person in charge of coordinating this research activity (Biosafety Officer) is Dane Ruscuklu (d.ruscuklu@nanogune.eu)
In the following link below you can find all the documents and work procedures that need to be read and filled PRIOR the introduction of the biological agents in the institute. After filling the document (biosafety_checklist) with all detailed information about the type of microorganism/cells and the working conditions, it has to be sent to the Biosafety officer to authorize the entry and the work with that bio agents.

Biological safety level (BSL) 1 & 2 laboratories
At nanoGUNE, we have facilities classified as BSL 1 and BSL 2 in which we can work with biological agents of these categories.
BSL1-enabled laboratories are Labs 7, 8, 9, 10, 15, 23 and Eukaryote Culture Room, while BSL 2 agents can only be handled in Laboratory 4 and in the Bacteria Culture Room (within the Culture Room Facilities).
The person in charge of coordinating this research activity (Biosafety Officer) is Dane Ruscuklu (d.ruscuklu@nanogune.eu)
In the following link below you can find all the documents and work procedures that need to be read and filled PRIOR the introduction of the biological agents in the institute. After filling the document (biosafety_checklist) with all detailed information about the type of microorganism/cells and the working conditions, it has to be sent to the Biosafety officer to authorize the entry and the work with that bio agents.

Sexual and Gender-Based Harassment
A Sexual and Gender-Based Anti-Harassment Protocol is in place at nanoGUNE, in order to (i) prevent sexual and gender-based harassment and (ii) act against it in case such an intolerable behavior occurs.
Confidential Counselors provide advice and assistance to anyone who may have suffered from sexual or gender-based harassment. A third person who knows about a harassment situation could also activate the protocol or ask advice to the confidential counselors.
Confidential counselors are Itziar Otegui (i.otegui@nanogune.eu | 943 574 024) and Laura Benedetti (l.benedetti@nanogune.eu | 943 574 049).
Sexual and Gender-Based Harassment
A Sexual and Gender-Based Anti-Harassment Protocol is in place at nanoGUNE, in order to (i) prevent sexual and gender-based harassment and (ii) act against it in case such an intolerable behavior occurs.
Confidential Counselors provide advice and assistance to anyone who may have suffered from sexual or gender-based harassment. A third person who knows about a harassment situation could also activate the protocol or ask advice to the confidential counselors.
Confidential counselors are Itziar Otegui (i.otegui@nanogune.eu | 943 574 024) and Laura Benedetti (l.benedetti@nanogune.eu | 943 574 049).
